See the Difference With Gleolan
Surgical Field Under White Light
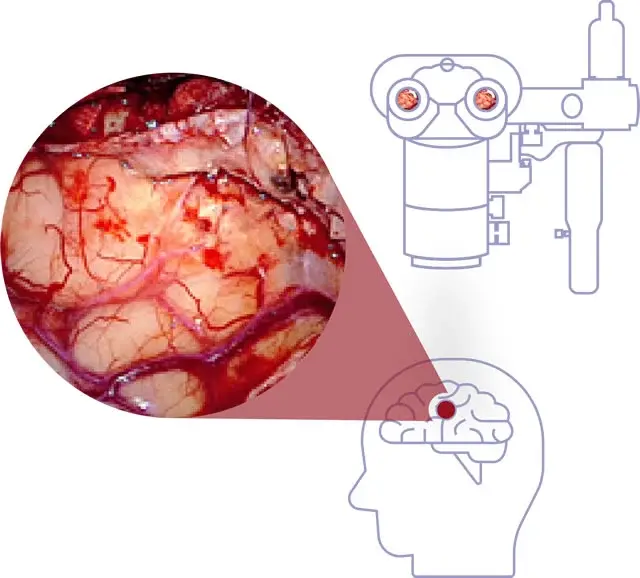
When illuminating the surgical field under white light, it may be difficult to distinguish the tumor from surrounding healthy tissue.1
Surgical Field Under Blue Filter and Fluorescing Tissue
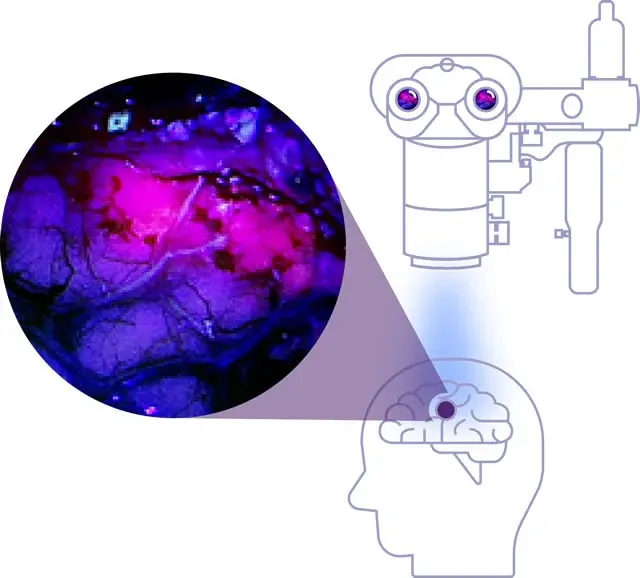
With Gleolan administered, when the surgical field is illuminated with white light using a blue filter, the high-grade glioma fluoresces red-violet, making it easier to identify.1
See How Gleolan Selectively Illuminates Glioma Cells
Gleolan is selectively taken up by receptors that are upregulated in glioma cells. Several enzymatic modifications lead to a build-up of fluorescence within the cells that glows red violet when viewed through a specific blue-light filter.
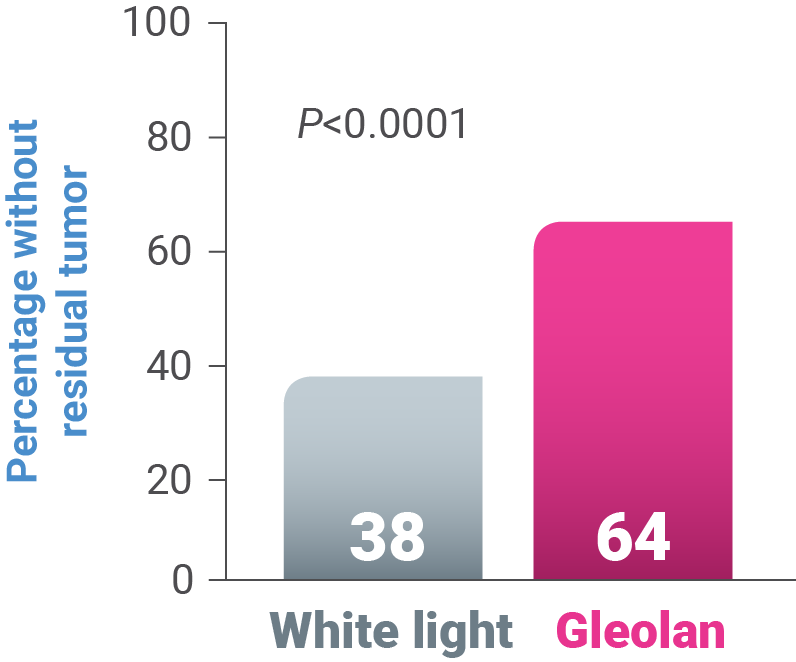
Gleolan Demonstrated Significant Improvement in EOR vs White Light1,3,4
-
64% of Gleolan patients had “completeness” of resection vs 38% in the control arm (P<0.0001)1,3,4
-
Surgical guidance with Gleolan is not limited by brain shift5
-
Gleolan may be used in adult suspected HGG patients of any age1
In a clinical study, patients with suspected malignant glioma amenable to complete resection of contrast-enhancing tumor were randomly assigned to 20 mg/kg Gleolan for fluorescence-guided resection (n=176) or to conventional microsurgery with white light (n=173). A primary endpoint was the number of patients without contrast-enhancing tumor on early MRI (within 72 hours after surgery for resection).3
Gleolan achieved >95% positive predictive value (PPV) for identification of malignant tissue across all studies.1,3,6,7
EOR, extent of resection; MRI, magnetic resonance imaging
See the Difference
- Indicated for use in suspected WHO Grade III and IV glioma1
- Surgeons using fluorescence-guided surgery with Gleolan demonstrated significant improvement in extent of resection vs white light1
- Facilitates real-time visualization of suspected WHO Grade III and IV glioma2
- Not limited by brain shift5
- >95% Positive Predictive Value (PPV) for identification of malignant tissue1,3,6,7
- NPV varies based on sampling location8
- Improved visualization, beyond that identified by preoperative MRI9
- Compatible with other technologies used during resection10
- Rates of long-term neurologic complications in procedures that used Gleolan fluorescence-guided surgery vs white light are similar4
Gleolan Was Generally Safe and Well-Tolerated
Supported by data from 5 open-label clinical trials on 527 patients1:
- Adverse reactions occurring in >1% of patients the week following surgery were pyrexia, hypotension, nausea, and vomiting1
- Easy-to-administer oral dosing 2-4 hours before anesthesia1
- Recognized in AANS/CNS, NCCN, and ESMO guidelines for the treatment of glioma11-13
- 5-ALA has been used in more than 100,000 patients in over 42 countries14
Due to risk of phototoxic reactions, do not administer phototoxic drugs (St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines) and topical preparations containing ALA for 24 hours during the perioperative period. Reduce exposure to sunlight or room lights for 48 hours after oral administration of Gleolan.
Please see Full Prescribing Information for additional important safety information.
REFERENCES
1. Gleolan Prescribing Information.
2. Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77(5):663-673.
3. Medical Imaging Drugs Advisory Committee. FDA Briefing Package; May 10, 2017. https://www.fda.gov/downloads/
4. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401.
5. Widhalm G. Intra-operative visualization of brain tumors with 5-aminolevulinic acid-induced fluorescence. Clin Neuropathol. 2014;33(4):260-278.
6. Stummer W, Tonn JC, Goetz, C, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74(3):310-320.
7. Nabavi A, Thrum H, Zountsas B, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009;65(6):1070-1077.
8. Díez Valle R, et al. J Neurooncol. 2011;102:105-113
9. Samkoe KS, Gibbs-Strauss SL, Yang HH, et al. Protoporphyrin IX fluorescence contrast in invasive glioblastomas is linearly correlated with Gd-enhanced magnetic resonance image contrast but has higher diagnostic accuracy. J Biomed Opt. 2011;16(9):096008.
10. Schucht P, et al. Neurosurgery. 2012;71:927-936
11. Patrick HH, Sherman JH, Elder JB, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of cytoreductive surgery in the management of progressive glioblastoma in adults. J Neurooncol. 2022;158(2):167-177. doi: 10.1007/s11060-021-03881-w
12. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. 2018. https://www.nccn.org/ professionals/physician_gls/pdf/cns.pdf
13. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G; ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii93-iii101.
14. Data on file, NX Development Corp.
Important Safety Information
Contraindications
Do not use Gleolan in patients with:
- hypersensitivity to aminolevulinic acid (ALA) or porphyrins
- acute or chronic types of porphyria
Warnings and Precautions
Due to the risk of phototoxic reactions, do not administer phototoxic drugs and topical preparations containing ALA for 24 hours during the perioperative period. Reduce exposure to sunlight or room lights for 48 hours after administration of Gleolan.
Errors may occur with the use of Gleolan for intraoperative visualization of malignant glioma, including false negatives and false positives. Non-fluorescing tissue in the surgical field does not rule out the presence of tumor in patients with glioma. Fluorescence may be seen in areas of inflammation or metastases from other tumor types.
Hypersensitivity reactions, including serious hypersensitivity reactions have occurred; these reactions include anaphylactic shock, swelling, and urticaria. Always have cardiopulmonary resuscitation personnel and equipment readily available and monitor all patients for hypersensitivity reactions.
Adverse Reactions
Adverse reactions occurring in >1% of patients in the week following surgery were pyrexia, hypotension, nausea, and vomiting.
Nervous system disorders occurred in 29% of patients within the first week after surgery and events occurring in >1% of patients included: aphasia (8%), hemiparesis (7.8%), hemianopsia (3.2%), headache (2.7%), seizure (1.9%), hemiplegia (1.9%), monoparesis (1.3%) and hypoesthesia (1.1%). Brain edema occurred in <1% of patients in the first 6 weeks after surgery. In a randomized clinical trial, the numbers of serious neurologic adverse events in the post operative period were higher in patients randomized to ALA fluorescence arm compared to the control arm. An imbalance was notable for the adverse events aphasia, ataxia, convulsion and hemianopsia and is likely related to the higher amount of brain resection performed in the ALA arm. At longer follow up periods, the numbers between the two arms appeared similar.
Worsening of >2 Common Toxicity Criteria grades in alanine aminotransferase and gamma-glutamyl transferase occurred in 15.8% and 11.6% of patients, respectively, within the first week after surgery. Absolute levels ranged from 2 times to greater than 10 times the upper limit of normal for each parameter. At 6 weeks, these measurements remained elevated in 2.9% and 7.5% of patients, respectively. There were no cases of liver failure.
Drug-Drug Interactions
See information under Warnings and Precautions regarding phototoxic reactions.
Please see Full Prescribing Information
For medical inquires, please fill out our Medical Information Request Form.

